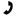An unknown alkene with molecular formula C7H12 undergoes the following reaction in basic KMnO4 under high heat to give one product (below). What is the identity of the alkene?

Loading ...
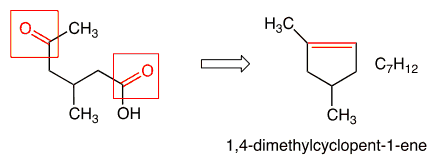
Correct Answer: E. 1,4-dimethylcyclopent-1-ene
The treatment of alkenes with acidic potassium permanganate under high temperatures results in oxidative cleavage. The nature of the products are dependent on alkene substitution, but all with possess a carbonyl functionality (carboxylic acid, ketone, CO2). Looking at the product, one can identify the two carbon atoms of the original alkenyl compound, and work back accordingly. Finally, standard IUPAC naming will apply.
Get it right? Tweet at us!
Subscribe below to get the DAT Question of the Day delivered straight to your inbox every morning.