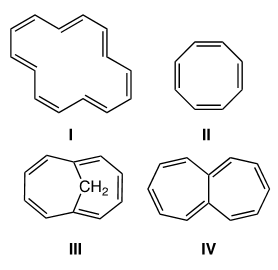
Which of the following compounds would be considered aromatic?

Loading ...
Correct Answer: D. I and III
Aromatic compounds follow four rules: (1) They are conjugated—there needs to one “p” orbital from each atom in the ring, so each atom must be either sp2 or sp hybridized; 2) They are cyclic: linear systems are not aromatic; 3) They are planar: there is good overlap/interaction between p orbitals; 4) they follow the The Huckel Rule—4n+2 π electrons in the cyclic conjugated π system (n = 0, 1, 2, 3 etc.). Of the possibilities,compounds I (14 e−, n = 3) and III (10 e−, n = 2) obey the rule.
This is a little advanced and at the top of the difficulty the DAT can throw at you, you have to consider compound 3 as one molecule, not two separate rings. Aromaticity requires a planar conjugated system, so we can ignore the bridge carbon sticking out of the plane of the rest of the molecule. There cannot be any sp3 atoms IN the aromatic ring. The carbon bridge actually pushes the 10 carbon ring to be planar, without it the molecule wouldn’t be aromatic.
Subscribe below to get the DAT Question of the Day delivered straight to your inbox every morning.

