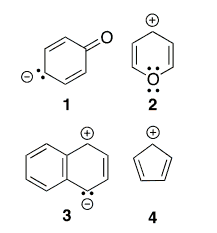
Which compound below does NOT show aromatic properties?

Loading ...
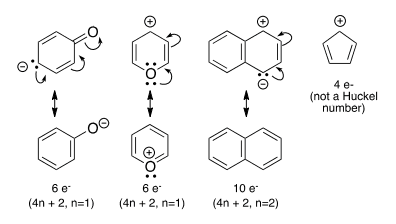
Correct Answer: D. 4
Aromatic compounds follow four rules: (1) They are conjugated—there needs to one “p” orbital from each atom in the ring, so each atom must be either sp2 or sp hybridized; 2) They are cyclic: linear systems are not aromatic; 3) They are planar: there is good overlap/interaction between p orbitals; 4) they follow the Huckel Rule — 4n+2 π electrons in the cyclic conjugated π system (n = 0, 1, 2, 3 etc.)
Get it right? Tweet at us!
Subscribe below to get the DAT Question of the Day delivered straight to your inbox every morning.

