For carbonyls that are part of a conjugated π-network, the C=O stretch:

Loading ...
Correct Answer: C. has a lower frequency than the analogous C=O in a non-conjugated system
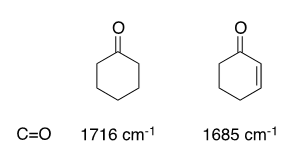
All carbonyl compounds absorb in the IR region of 1760–1665 cm–1 owing to the stretching vibration of the C=O bond. This carbonyl band is distinct with a characteristic high intensity, and therefore is quite useful for diagnostic purposes because few other functional groups absorb in this region. Conjugation with a double bond or benzene ring lowers the stretching frequency, usually by 30 to 40 cm–1. The stretching frequency of the conjugated double bond is also lowered and may be enhanced in intensity. An example of this phenomenon is provided:
Subscribe below to get the DAT Question of the Day delivered straight to your inbox every morning.