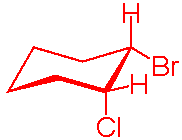
The most stable conformational isomer of cis-1-bromo-2-chlorocyclohexane involves:

Loading ...
Correct Answer: C. The bromine atom in the equatorial position and the chlorine atom in the axial position.
The bromine atom is heavier and larger so it belongs in the equatorial position. Generally, the larger atoms belong in the equatorial position to minimize the steric strain. Having the bromine point away from the ring is better than having it point in an axial direction, where it will meet resistance and strain from other hydrogen atoms.
Note that the problem says this is a cis isomer, and therefore answers A, B, and E are impossible when you draw out the chair conformation. If the atoms are on the same side of the molecule, then one must be equatorial and the other axially. For stabilization purposes, it is better for the larger bromine atom to be in the equatorial position and the chlorine in the axial position.
Subscribe below to receive the DAT Question of the Day delivered straight to your inbox every morning.