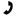Consider the molecule below. If the CH3- and Cl- groups were switched with each other, how would you describe this new molecule relative to the one below?

Loading ...
Correct Answer: E. diastereomer.
Drawing this new molecule (right) and comparing it to the first (left), we see that the two are stereoisomers of each other, specifically, diastereomeric. They are not mirror images and non-superimposable (conditions for enantiomers). Constitutional isomerism does not apply here, as connectivity of atoms remains the same. Rotation about the bond drawn in stick form does not yield identical compounds (not rotamers).
Subscribe below to get the DAT Question of the Day delivered straight to your inbox every morning.