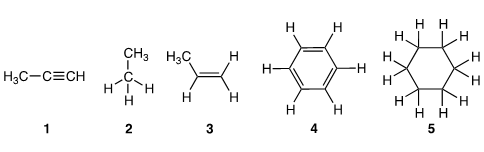
Which of the following would you expect to be the strongest acid?

Loading ...
Correct Answer: A. 1
Simple hydrocarbons (containing only C and H) are weakly acidic in comparison to other organic compounds. However, carbanions can be formed, with their stability depending quite heavily on the hybridization state of the orbital containing the lone pair of electrons. Alkyne 1 is most acidic as the lone pair of the conjugate base is held in a sp-orbital, rather than sp3 (for 2 and 5) or sp2 orbital (for 3 and 4). Having a greater proportion of “s character” allows for the electron density in an sp-orbital to be held closer to the positively charged nucleus, making it electrostatically more stable.
Subscribe below to get the DAT Question of the Day delivered straight to your inbox every morning.

