Consider the following aqueous equation:
6 NaI + Ca3(PO4)2 -> 2 Na3PO4 + 3 CaI2
If 14 moles of NaI, 8 moles of Ca3(PO4)2, and 6 moles of Na3PO4 are present in a mixture, which one of the following compounds would determine the amount of CaI2 that was produced after 2 hours?

Loading ...
Correct Answer: A. NaI
This is a limiting reagent question. Only one of the reactants can “limit” the amount of CaI2 produced, therefore answer options [C] and [D] are eliminated. Even though the compounds are aqueous, dissolved in water, water is not written in the equation and therefore should not have an effect on the amount of product produced, thus eliminating answer option [E].
Next, we setup two equations to see the amount of CaI2 both reactants can make. The reactant that makes the SMALLEST amount of CaI2 is our limiting reagent.
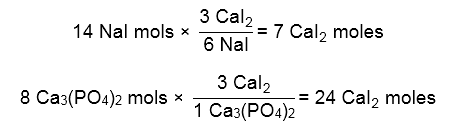
NaI produces less mols of our product, so it must be the limiting reagent.
Subscribe below to get the DAT Question of the Day delivered straight to your inbox every morning.