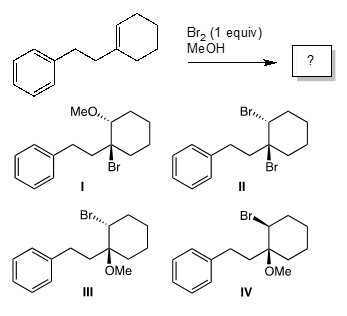
What is the major product of the following reaction?

Loading ...
Correct Answer: C. III
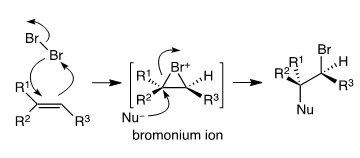
The bromonium ion is formed when alkenes react with bromine. When the π cloud of the alkene (nucleophile) approaches the bromine molecule (acting as an electrophile), the σ-bond electrons of Br2 are pushed away, resulting in the departure of the bromide anion. The species formed is a high-energy intermediate along the reaction path. It will react further with some nucleophile−methanol in this case. The electronic structure of the bromonium ion has its consequences in the stereochemistry of the reaction. Nucleophilic attack takes place from the bottom face (back-side to the “departing” bromide) on the more substituted carbon. The back-side attack leads to the anti addition (Br and Nu groups end up on the opposite faces of the starting double bond), and the nucleophile adds to the more substituted carbon (Markovnikov’s rule). Structure III corresponds to the above. Structure II may form, but only in very miniscule amounts.
Subscribe below to get the DAT Question of the Day delivered straight to your inbox every morning.

