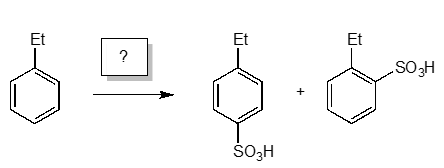
Which reagents would you use to transform ethylbenzene to the following products?

Loading ...
Correct Answer: C. SO3 / H2SO4 / heat
The sulfonation of ethylbenzene requires fuming sulfuric acid and sulfur trioxide. Reaction A would not give the product; reaction B would result in nitration rather than sulfonation; reaction D woul result in oxidation of sulfurous acid to sulfuric acids by peroxides, but without other reagents, would not react further. Note that the para isomer would be the major product for steric considerations.
Subscribe below to get the DAT Question of the Day delivered straight to your inbox every morning.

