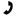Determine the structure of an unknown compound with the molecular formula C8H12O that has 5 signals in the H NMR spectrum and shows the following IR absorptions:

Loading ...
Correct Answer: A. I
Of the possibilities, all have the molecular formula C8H12O, but only compounds I and III can have 5 chemically unique proton signals in the H NMR spectrum. The broad IR peak at 3400 (indicative of alcohol), and sharp peak at 3300 (alkyne C-H stretch) confirms the compounds identity as I.
Get it right? Tweet at us!
Subscribe below to get the DAT Question of the Day delivered straight to your inbox every morning.