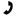Mixture A is formed using KCl and H2O and mixture B is formed using MgCl2 and H2O. If the two mixtures have the same molality, which of the following statements would be true about the boiling point of the mixtures?

Loading ...
Correct Answer: B. Mixture A would have a lower boiling point
The two mixtures have the same concentration a.k.a. molality. However, they do have differing ionic solutes. Mixture A has an ionic solute that would dissociate to form two ions (K+ and Cl–) whereas Mixture B has an ionic solute that forms three ions (Mg2+ and 2Cl–). In the equation:
∆T(boiling) = m x Kb x i, the “I” or vant Hoff factor would be the only variable. The higher the vant Hoff factor, the greater the effect on the boiling point and/or freezing point. The von hoff factor for Mixture A is 2, but for Mixture B is 3. Therefore, Mixture B would have the greater affect on the boiling point, making it higher than normal. Between the two mixtures, B would have a higher boiling point than mixture A.
Subscribe below to get the DAT Question of the Day delivered straight to your inbox every morning.