The forces responsible for the relatively high melting point and boiling point of water, H2O, are:
Subscribe below to get the DAT Question of the Day delivered straight to your inbox every morning.
The forces responsible for the relatively high melting point and boiling point of water, H2O, are:
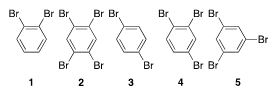
A reagent bottle with the label “brominated benzene” shows two peaks in the carbon NMR spectrum. Which of the following are possible structures for the mystery compound?
One of the tanks that a pediatric dentist uses to administer nitrous oxide is “empty”, meaning that the N2O inside is at the same temperature and pressure as the room. The ML-6 type cylinder has an internal volume of 165 liters. How many moles of nitrous oxide remain in the empty tank (Ideal gas constant = 0.082 L atm/K mol; assume the apparatus is at sea level; the ambient temperature is 22° C)?
Photo attributed to Jurvetson.
In a reaction between Mg and Ar, which of the following is most likely to occur?
Which of the following is at the highest energy during an endothermic chemical reaction?
Which of the following elements would contain the valence electron configuration 4s24p5?
Which of the following molecules has a bond that is nearest to a pure covalent bond?
Photo attributed to Rudolf_Schuba.
For a mixture of gases in a container, the total pressure is equal to __________ because of what law?
The enthalpy of a system of gas is dependent on which of the following?
The hardest substance known is diamond. Which best describes the structure of diamond?